Denisovan HLA and its role in immunity
Today's people have inherited several immune alleles from archaic people. Understanding what difference they made is a continuing challenge.
Everything I write about function in archaic genomes starts with immune phenotypes. As early as 2006, my research was focused on understanding how adaptive gene variants from ancient populations like the Neanderthals may have been important to the evolution of later people. Even in that early work, I saw that immune system genes were among the best candidates for today’s people to have inherited from archaic groups.
I was drawing my comparisons from nonhuman species like cattle, in which geneticists had documented gene flow across distantly related populations—a phenomenon called introgression. Some of the most common introgressed genes were involved in the immune system of those animals.
It’s easy to see why: Pathogens often find ways to hop across natural boundaries, even the boundaries between species. When they do, the pathogens’ new hosts may benefit from gene variants that evolved in other populations that coevolved with similar pathogens.
When Svante Pääbo’s research team first recovered ancient DNA of Neanderthals and Denisovans, established researchers in immunogenetics quickly started looking at these genomes. One of the first studies to investigate possible functional effects of Denisovan and Neanderthal genes was published in 2011 by Laurent Abi-Rached and collaborators, who looked at the large family of genes known as the human leukocyte antigen (HLA) complex. In other vertebrates, these genes aren’t called “human”—instead they’re known as the major histocompatibility complex (MHC).
I’m coming to the stage of writing in my current book project that I have a lot of background research to share. I thought I would start by sharing some things about Denisovan DNA and its role in immunity.
The role of HLA
The immune system is complicated. A human genome has more than 20,000 protein-coding genes, and research has connected more than 1500 genes to immune responses. That’s at least seven percent of the genome.
This complexity evolved not just during the seven-million-year evolution of hominins but across the hundreds of millions of years that vertebrates have diversified. Today immune systems help humans survive more than 1000 viruses, bacteria, fungi, and parasites that infect people around the world.
Most of the body’s cells use HLA class I molecules to help transport and display small fragments of proteins known as peptides on their cell membranes. One class of immune T cells, known as cytotoxic (CD8+) T cells, interact with the HLA class I molecules. When they detect a foreign antigen, like the kinds that are produced by viruses, the CD8+ T cells kill the infected cell.
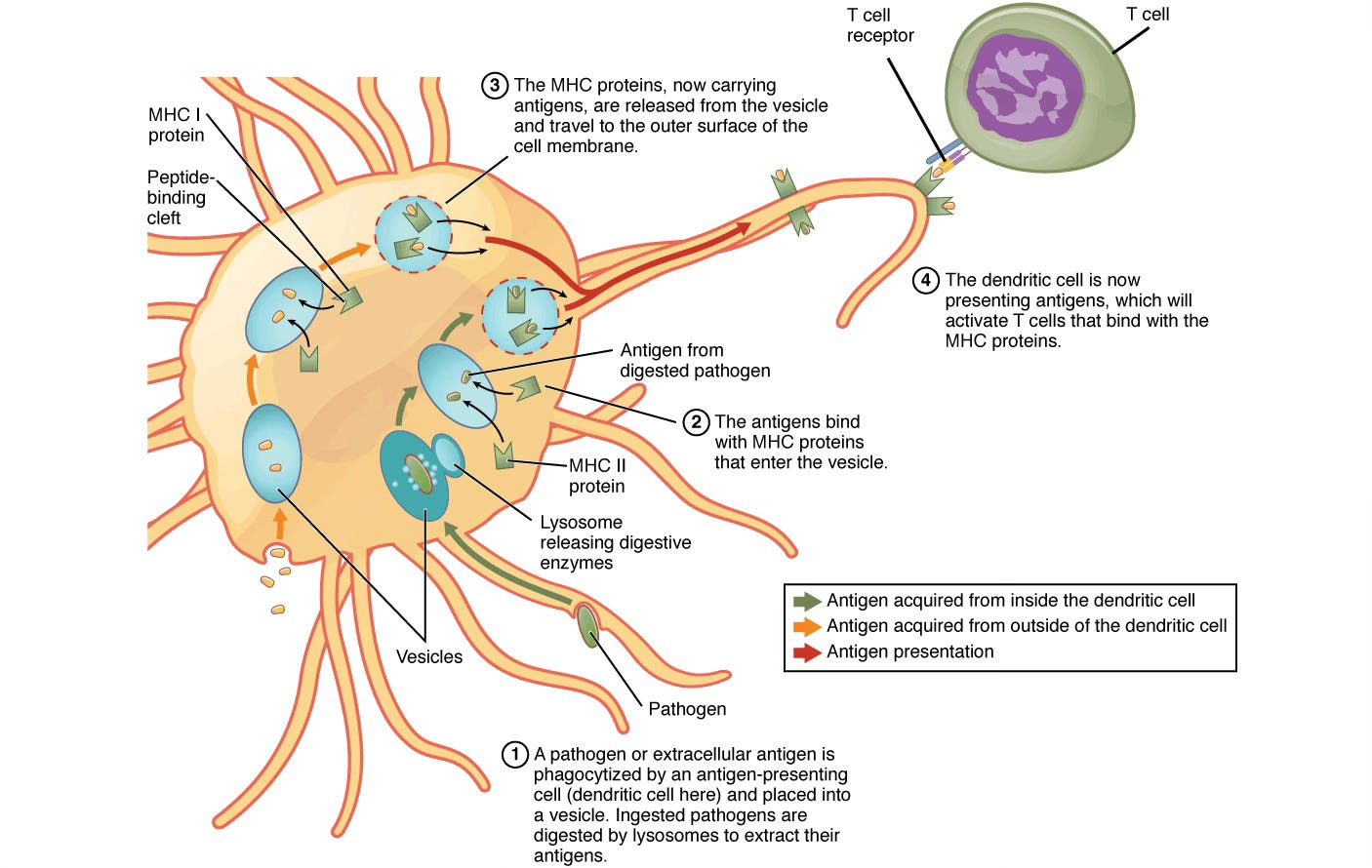
Another class of immune cells, known as antigen-presenting cells (APCs), specialize in taking peptides from outside of cells. They combine these peptides with HLA class II molecules, and then present them to CD4+ T cells, the “helper” T cells. The “help” is that they activate another kind of cell, known as B cells, to produce antibodies. Antibodies are large Y-shaped proteins that can bind directly to viruses and bacteria, inhibiting them from infecting cells. Antibodies also tag the pathogens to help large immune cells called macrophages to engulf and destroy them.
Diversity is central to the function of the HLA system: Each of the genes has many alleles in human populations, and individuals with rare alleles sometimes have a big advantage when new pathogens strike, a pattern known as frequency-dependent selection. Having two different alleles for an HLA gene can sometimes provide dual protection against different pathogens, called heterozygote advantage. Both these patterns are known as balancing selection, and they can favor new alleles and haplotype combinations that gene flow brings into a population.
That includes genes from archaic human populations.
Studying haplotypes
For a particular gene, such as HLA-A, the different forms that can be detected are usually called alleles. The different genetic states of any polymorphism in DNA—including single nucleotide polymorphisms—likewise are usually called alleles.
Nearby alleles on a single copy of a chromosome are generally inherited together, unless recombination during meiosis has happened between them. The coinheritance of nearby alleles means that geneticists can identify long chromosome segments transmitted over time through family trees, known as haplotypes. Haplotypes can span one, several, or even dozens of genes. They get shorter over time as recombination whittles them away.
DNA from archaic people is divided into a patchwork of haplotypes, that vary in length from 20,000 base pairs up to a million base pairs or more. Most of these chunks are rare today but a few have become quite common.
The fraction of Denisovan ancestry across the whole genome maxes out at around 4% in some indigenous groups of the Philippines, and up to 3% in the indigenous peoples of Papua and Australia. In mainland East Asia, this fraction is much smaller, only around 0.2%. For other Eurasian populations, the amount is even less, often 0.1% or smaller.
Even that tiny fraction still adds up to 6 million base pairs across a genome. But as the fraction gets smaller and smaller, it’s very hard to be sure that DNA comes from one archaic group and not another. Each of the potential ancestral groups was itself diverse. That means incomplete lineage sorting within the ancient African population or ancestry from Neanderthals might resemble Denisovan ancestry. In the case of HLA variation, balancing selection was also elevating diversity in each of the ancestral groups.
Denisovan HLA variation
In their early work, Abi-Rached and coworkers highlighted HLA haplotypes in today’s humans that may have come from Neanderthals, Denisovans, or potentially unknown groups. Their ability to assess HLA haplotypes in archaic genomes was limited by the highly fragmented state of ancient DNA. Still, they could look for allelic differences within or nearby specific HLA genes and suggest which human HLA haplotypes match the archaic DNA.
In the Denisova 3 genome, they suggested that the HLA-A*02 allele and the HLA-C*12:02 allele were present. These two alleles appear in linked form as a combined haplotype today in Papua New Guinea and adjacent areas. The other alleles that seemed to be present in the Denisova 3 genome are HLA-A*11, a common today in East and Southeast Asia, and HLA-C*15, common in Papua New Guinea and Australia.
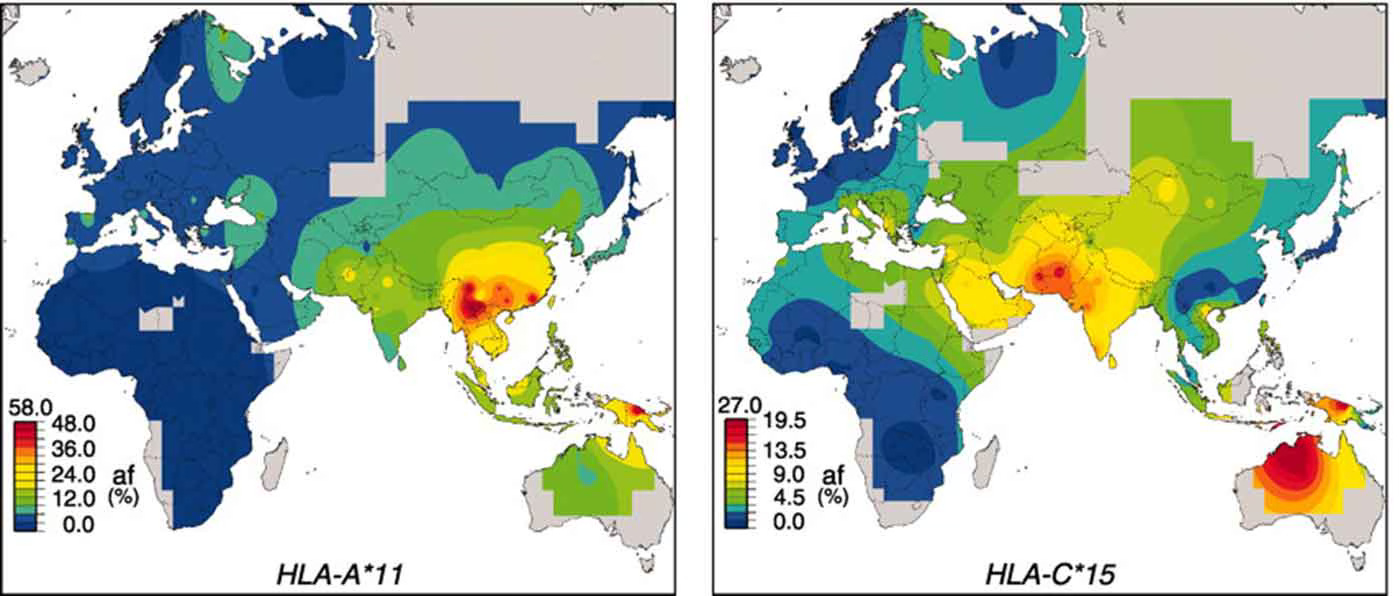
The linkage of alleles upon longer combined haplotypes is an important part of the biology of the HLA complex in humans. It can make studying these genes fiendishly difficult.
I remember tracing across the first Denisovan genome on my computer screen, looking at sequence polymorphisms in today’s genomes to see if they were covered by the short fragments of Denisova 3 sequence. My then-PhD student Aaron Sams, whose dissertation work focused on the evolutionary history of autoimmune diseases in humans, spent a lot of time with HLA and archaic genomes. It proved to be a big challenge. Specific identification of alleles is very hard to achieve for ancient genomes with their large number of gaps and missing data.
Those limitations have shaped the history of research on archaic HLA. For example, Abi-Rached and coworkers proposed that the HLA-B*73 haplotype was a strong candidate for Denisovan ancestry. Their idea did not come from the key mutations that define HLA-B*73 itself, but from linked mutations that are often part of the same haplotypes as HLA-B*73 in living people. Later work has suggested that HLA-B*73 was likely passed down from the African ancestors of today’s people, not Denisovans, although it is still possible that some linked haplotypes had a Denisovan origin.
But with HLA genes from Denisovans, the lack of ancient sequences remains a fundamental limit on our knowledge of variation in the past. There may be many HLA haplotypes across East and Southeast Asia, or even into the Americas, Europe, and the Pacific, that we might recognize as Denisovan if the DNA of southern representatives of that population were known.
Frustrated about function
The search for archaic DNA's role in our immune system is a frustrating puzzle. We can find a lot of associations for specific alleles in modern people affected by disease, but we rarely know how these ancient DNA fragments helped our ancestors fight off ancient pathogens. While we have many ideas about what Denisovan HLA genes do, very few studies can pinpoint which pathogens they actually fought or how they worked.
Part of the problem is the sheer number of possible HLA alleles. HLA molecules hold out peptides for the rest of the immune system to see. With dozens of different HLA alleles and thousands of different pathogen peptides, it's very hard to figure out which combination is most important for a disease without studying a huge number of people.
A bigger problem is a shortfall of investment and investigation of both human populations and their pathogens.
Today, the people with the most Denisovan DNA live in island Southeast Asia and Oceania. But historically, these regions have been overlooked in genetic studies, with fewer than 1% of participants in genome-wide studies coming from these areas.
Meanwhile, the diseases that may have most affected ancient people are also understudied. Some are so underrepresented in medical research that they are officially known as “neglected tropical diseases”. Only a trickle of research dollars goes into conditions like yaws or scrub typhus, or into parasitic conditions like filariasis and roundworm. These and other diseases that are persistent in soil, foods, or fecal material would have been sustained over time by repeated reinfection in small-scale societies. But their interactions with the immune system are less well-known than many diseases that are more important in industrialized nations of Europe, East Asia, and North America.
What this all means is that the discussion section of papers about Denisovan HLA (and immunity more broadly) usually have statements that look a lot like arm-waving.
“HLA-A*11, an abundant archaic allotype in modern Asian populations, provides T cell–mediated protection against some strains of Epstein-Barr virus (EBV), and in combination with a peptide derived from EBV, is one of only two HLA ligands for the KIR3DL2 NK cell receptor.”—Laurent Abi-Rached and coworkers
It’s valuable to note such indirect evidence about possible functional effects of Denisovan alleles. The Epstein-Barr virus in particular may have had impacts on past human groups. But what’s frustrating is how little else can be done with today’s datasets. Even in this case, exactly how the course of EBV may have been affected by the possible Denisovan allele is unclear.
Some research groups are taking a broader look at the function of haplotypes from archaic people by studying their effects on gene regulation.
This is exactly the kind of investigation needed. In a recent study, researchers led by Danat Yermakovich found a strong link between Denisovan HLA haplotypes and adaptation to lowland environments in Papua New Guinea. They studied the genomes of over 50 people from the highlands and more than 70 people from a coastal island. The island group, which has likely faced different pathogens, had a much higher frequency of Denisovan haplotypes, particularly in the HLA region.
“Our findings imply that Denisovan DNA may have played a role in the adaptation to defense of malaria and/or other tropical diseases.”—Danat Yermakovich and collaborators
It’s a reasonable explanation for this pattern that diseases of the coastal lowlands may have selected for greater immune diversity.
The research into archaic DNA's role in our immune system is still in its early stages. To fully understand how ancient genes matter today, we need to broaden our focus. This means studying more of the world’s diverse populations that are underrepresented in recent research. It also means investing in research on the infectious diseases that have shaped human evolution for millennia—even if they aren’t the hot topics for research in the global north. The answers to some of the biggest questions about human health may not be found in our cities, but in the echoes of ancient migrations and the enduring battles against diseases of the distant past.
As scientists continue to explore these genetic whispers from the past, we're not just uncovering the secrets of an extinct human relative. We're finding clues about our own health, revealing the deep and complex connection between our long-gone ancestors and the challenges our bodies face today.
References
Abi-Rached, L., Jobin, M. J., Kulkarni, S., McWhinnie, A., Dalva, K., Gragert, L., Babrzadeh, F., Gharizadeh, B., Luo, M., Plummer, F. A., Kimani, J., Carrington, M., Middleton, D., Rajalingam, R., Beksac, M., Marsh, S. G. E., Maiers, M., Guethlein, L. A., Tavoularis, S., … Parham, P. (2011). The Shaping of Modern Human Immune Systems by Multiregional Admixture with Archaic Humans. Science, 334(6052), 89–94. https://doi.org/10.1126/science.1209202
Comerford, M., Vespasiani, D. M., Shukla, N., Cook, L. E., Yermakovich, D., Dannemann, M., Leavesley, M., Kinipi, C., Ricaut, F.-X., Brucato, N., Cox, M. P., & Romero, I. G. (2025). Mapping the gene regulatory landscape of archaic hominin introgression in modern Papuans (p. 2025.05.04.652069). bioRxiv. https://doi.org/10.1101/2025.05.04.652069
Fu, Q., Cao, P., Dai, Q., Bennett, E. A., Feng, X., Yang, M. A., Ping, W., Pääbo, S., & Ji, Q. (2025). Denisovan mitochondrial DNA from dental calculus of the >146,000-year-old Harbin cranium. Cell, 0(0). https://doi.org/10.1016/j.cell.2025.05.040
Gokcumen, O. (2020). Archaic hominin introgression into modern human genomes. American Journal of Physical Anthropology, 171(S70), 60–73. https://doi.org/10.1002/ajpa.23951
Hawks, J. & Cochran, G. (2006). Dynamics of adaptive introgression from archaic to modern humans. PaleoAnthropology, 2006, 101–115.
Hawks, J., Cochran, G., Harpending, H. C., & Lahn, B. T. (2008). A genetic legacy from archaic Homo. Trends in Genetics, 24(1), 19–23. https://doi.org/10.1016/j.tig.2007.10.003
Ongaro, L., & Huerta-Sanchez, E. (2024). A history of multiple Denisovan introgression events in modern humans. Nature Genetics, 56(12), 2612–2622. https://doi.org/10.1038/s41588-024-01960-y
Patin, E., & Quintana-Murci, L. (2025). Tracing the Evolution of Human Immunity Through Ancient DNA. Annual Review of Immunology, 43(Volume 43, 2025), 57–82. https://doi.org/10.1146/annurev-immunol-082323-024638
Vespasiani, D. M., Jacobs, G. S., Cook, L. E., Brucato, N., Leavesley, M., Kinipi, C., Ricaut, F.-X., Cox, M. P., & Romero, I. G. (2022). Denisovan introgression has shaped the immune system of present-day Papuans. PLOS Genetics, 18(12), e1010470. https://doi.org/10.1371/journal.pgen.1010470
Yermakovich, D., André, M., Brucato, N., Kariwiga, J., Leavesley, M., Pankratov, V., Mondal, M., Ricaut, F.-X., & Dannemann, M. (2024). Denisovan admixture facilitated environmental adaptation in Papua New Guinean populations. Proceedings of the National Academy of Sciences, 121(26), e2405889121. https://doi.org/10.1073/pnas.2405889121

